Can Potassium Form a Covalent Bond
Potassium nitrate is KNO3. How many bonds can potassium make and why.
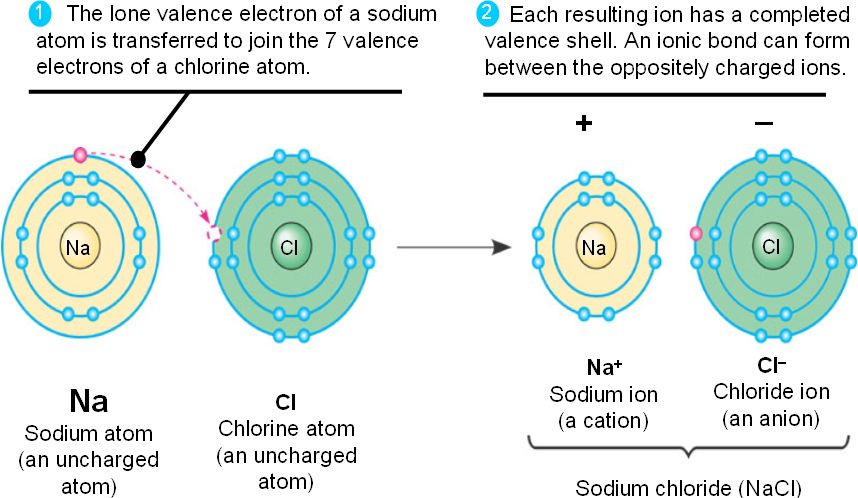
Is Potassium Iodide An Ionic Or Covalent Compound Gufosaggio
Only an ionic bond.

. Why are mathrmNa and mathrmK unable to form covalent bonds 0113. These electron pairs are known as shared pairs or bonding pairs and the stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding. Nonmetals can form different types of bonds depending on their partner atoms.
A covalent is formed when two atoms that tend to gain electrons are brought into contact with each other. Only an ionic bond. There we go that has a covalent bond between the nitrogen and oxygen and an ionic bond between potassium and the nitrogen and oxygen.
Is potassium chloride ionic or covalent. For the second one potassium chloride is the answer because the other three also have covalent bonds. Potassium does not form covalent bonds.
Explain why argon does not form either a ionic bonds or b covalent bonds 0052. Nonmetals are elements that can be. Potassium can form two bonds as it has one valence electron.
However it is assumed that the. Answer 1 of 5. Consider whether each element is a metal or a nonmetal to determine the sort of bond that forms between them.
Although non-polar molecular iodine cannot dissolve in water it reacts with iodide ion to form something that can. Covalent bond A covalent bond also called a molecular bond is a chemical bond that involves the sharing of electron pairs between atoms. Potassium chloride is KCl.
Elements can be classified as either metals or nonmetals based on their behavior in acid solutions. According to it the metallic bond is treated essentially as Covalent in character. Why is lattice energy the key to forming an ionic bond.
Ionic is formed when an atom that tends to lose electrons makes contact with an atom that tends to gain them. Does potassium form covalent bonds. Atoms form covalent bonds in order to reach a more stable state.
Ionic bond and covalent bond what is the difference. Explain why potassium does not bond with neon to form a compound. Because of its low electronegativity and the fact that it has only one electron in its valence cell it forms a cation with.
It can form a single covalent bond or it can form an ionic bond. In general nonmetals form covalent bonds metals and nonmetals create ionic connections and metals form metallic bonds. LPauling has tried to explain the metallic bond according to Valence bond theory.
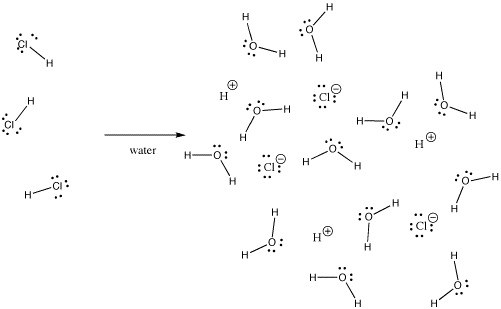
Na being metal has metallic bond. Potassium hydride is KH. Potassium iodide an ionic compound dissolves easily in water but does not dissolve in chloroform and hexane.
Ionic bonds form when a nonmetal and a metal exchange electrons while covalent bonds form when electrons are shared between two nonmetals.

Solved Explain Why Potassium Does Not Bond With Neon To Form A Compound
Example 4 Potassium Nitride Potassium Has One Valence

This Is How The Ionic Bond Forms In Potassium Chloride Kcl Youtube
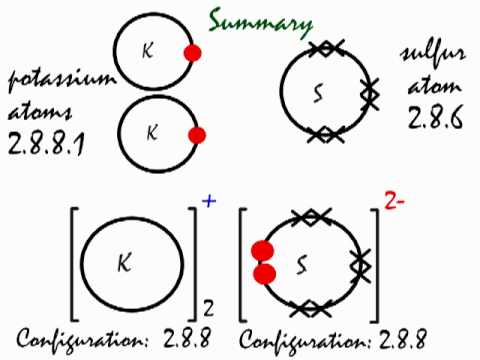
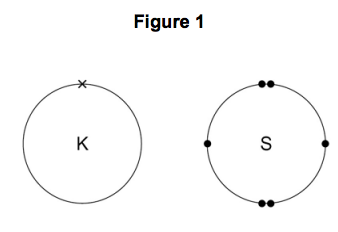
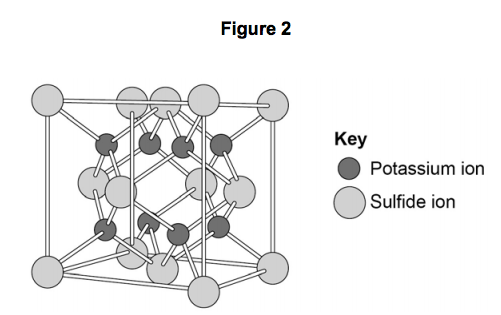
This Is How The Ionic Bond Forms In Potassium Sulfide K2s Youtube
How Is Potassium Chloride An Iconic Bond Quora
How Is Potassium Chloride An Iconic Bond Quora

Homework 1 Purple Due Monday 4 15 Green
What Is The Correct Formula For The Ionic Compound Formed Between Potassium And Oxygen Quora

What Type Of Chemical Bond Is Formed Between A Potassium And Bromine B Carbon And Bromine Youtube

Example 4 Potassium Nitride Ppt Download

Is Kcl Covalent Or Ionic Techiescientist

Is K2o Potassium Oxide Ionic Or Covalent Youtube

Example 4 Potassium Nitride Ppt Download
Example 4 Potassium Nitride Potassium Has One Valence


Comments
Post a Comment